Contact us now for a no-obligation exchange.

Servinvent.
The digital platform for simple, cost-effective and fast recording and management of any devices and objects.
As a manufacturer, reseller or operator, you can leverage the Servinvent platform to digitally manage medical devices in compliance with MDR (Medical Device Regulations) and coordinate their maintenance. The cloud-based solution aids inventorying and informs you when a device has reached the end of its lifecycle.
Current situation.
New regulations such as MDR (Medical Device Regulation) place greater obligations on manufacturers, resellers and operators in the medical sector. Operators must ensure that devices are maintained and documented according to the manufacturer's specifications. Today, the management of device data and the historisation of maintenance work is mainly done by manually entering and maintaining data records in static lists and databases. Although the management of devices is very often offered in modern ERP, these are usually very complicated in structure, and the recording and maintenance of this data is extremely time-consuming and complicated.
Servinvent highlights.
- Support in the implementation of regulations such as MDR and others
- Management of any device types and their documentation
- Recording of device type-specific maintenance cycles
- Historisation of maintenance work
- Coordination of inspections, maintenance, repairs and End-Of-Life
- Interface import / export of equipment data from ERP systems to Servinvent and vice versa
- Generation of QR codes for the identification of devices
- Scanning of devices with QR and data matrix codes
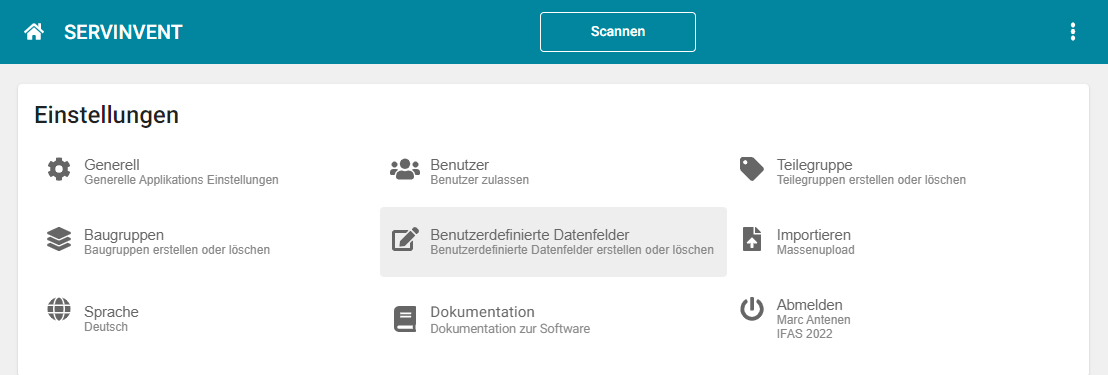
- Separate access for manufacturers, resellers and operators
- User and rights management
- Can be used as a complement to existing solutions
- Access via mobile and desktop devices
Solution.
Servinvent is very popular today, especially in the medical environment, because of its ease of use and the possibility of implementing the MDR requirements (Medical Device Regulation). Hospitals, nursing homes, physio practices, dental practices, laboratories and veterinary practices have discovered the software for themselves.
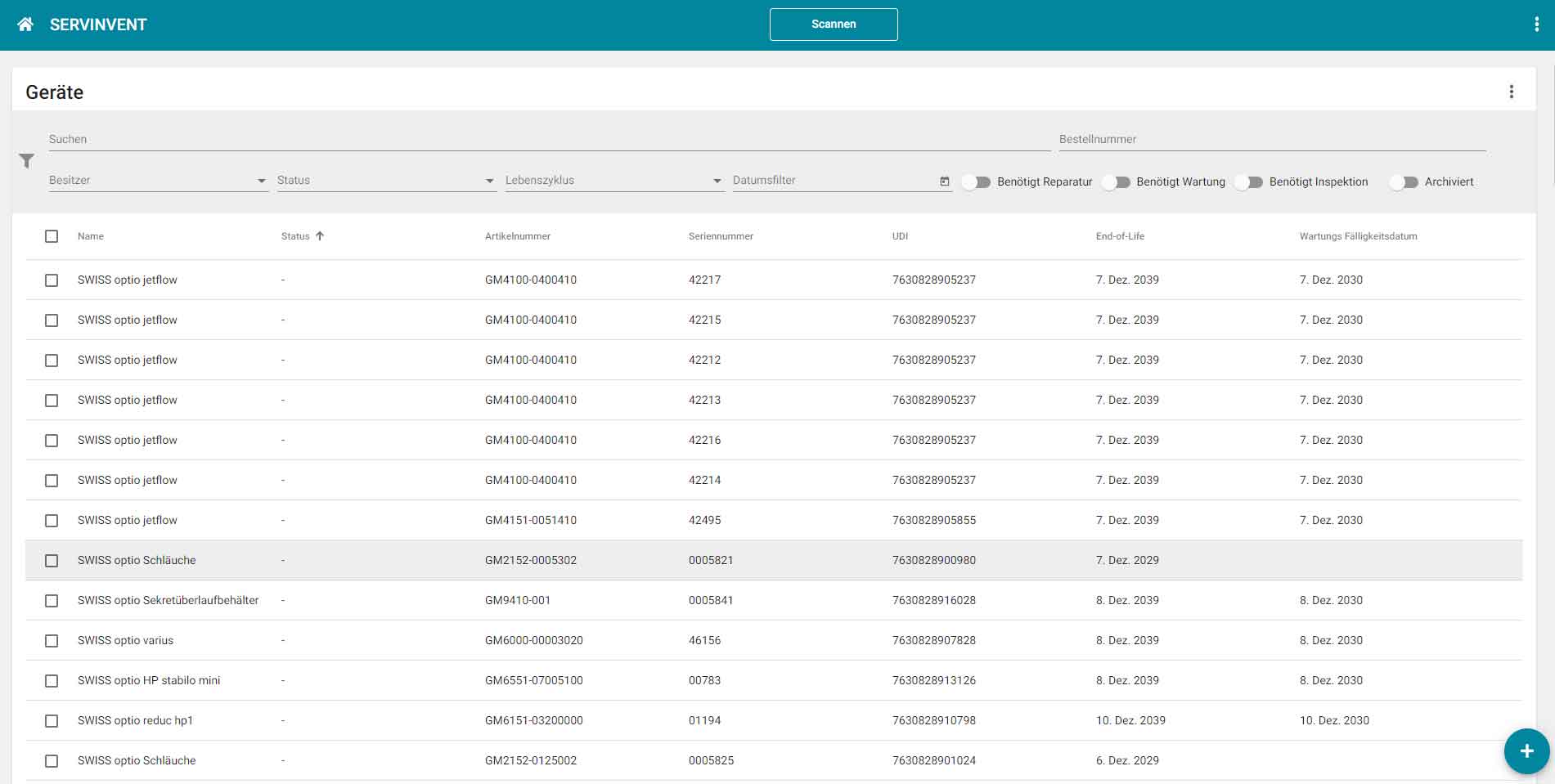
The industry uses the software to record and manage machines, tools, vehicle fleets, measuring instruments and test equipment of all kinds, for simply all items with a maintenance and cleaning cycle, including certificate and documentation management.
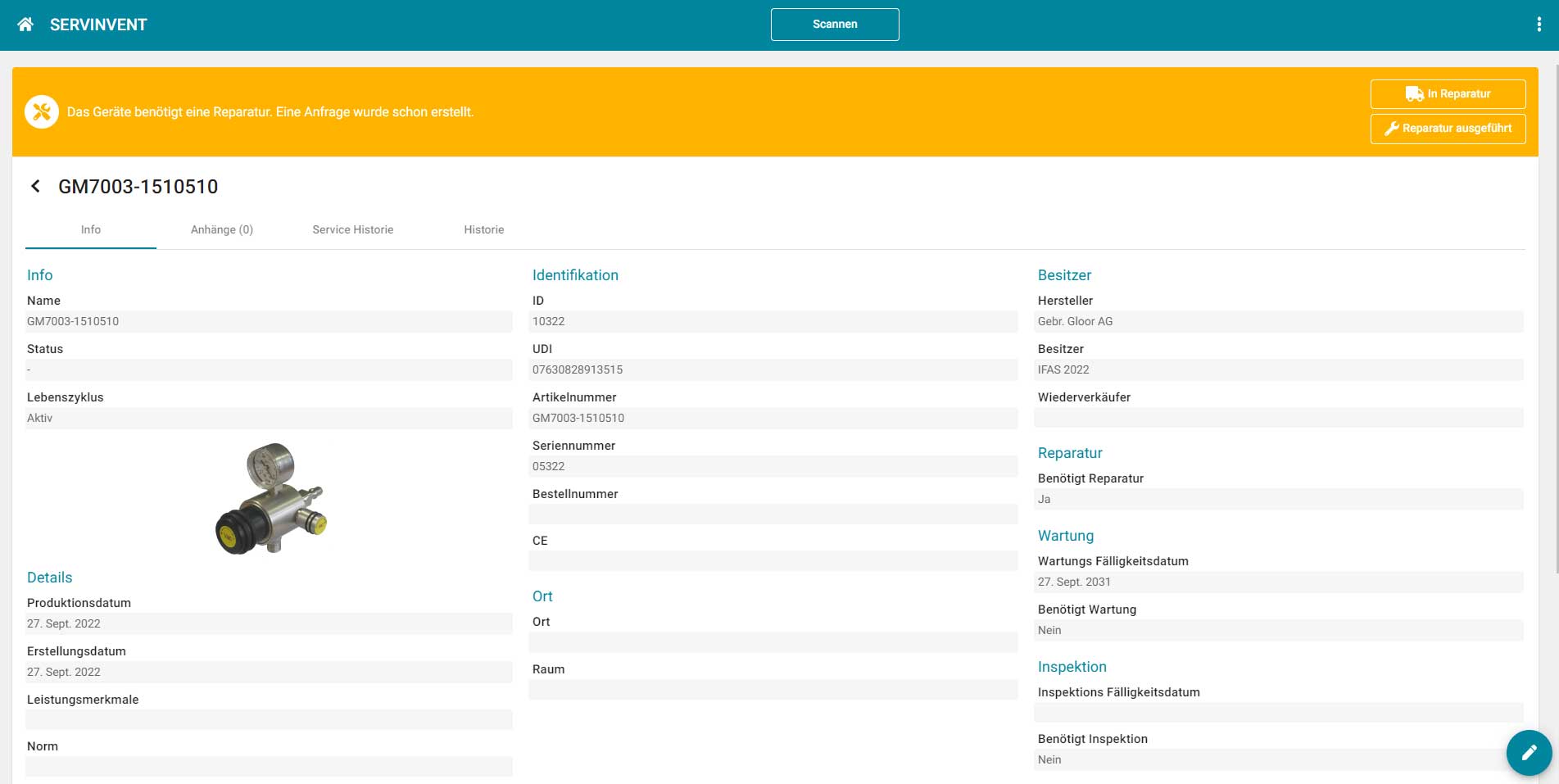
The web-based platform "Servinvent" can be used without installation on any device with a web browser. Data can be automatically imported from ERP systems via interfaces or entered directly using the Servinvent user interface.
Users receive an overview of all devices and can display upcoming or due maintenance dates. Service technicians have the option of scanning devices using QR or Datamatrix codes via mobile (smartphone or tablet) and recording the maintenance carried out directly via the Servinvent platform or retrieving the required instructions, manuals and certificates.
Why Bechtle?
As a leading IT service provider in Switzerland, we, Bechtle Schweiz AG, are the partner of choice for SMEs, large customers and public institutions for consulting, IT infrastructure, cloud solutions, IT services and software.
Our offer covers the entire IT lifecycle, from consulting to implementation and operation. Our customers benefit from the highest partner certifications with most well-known manufacturers.
Why Gloor?
As an innovative SME and renowned manufacturer of medical fittings and gas sampling devices in the medical market, we know the Swiss hospital landscape extremely well. The needs of our customers, especially with regard to device management and / or various product maintenance issues, occupy us on a daily basis. We accompany and support our customers in these complex projects in a solution-oriented and practical manner.


If you’d like to know more about how we handle your personal data, please read our Privacy Policy.